Introduction: Fanconi anemia (FA) is a rare, genetic disorder clinically characterized by congenital abnormalities, progressive bone marrow failure (BMF), and a predisposition to malignancies. Allogeneic hematopoietic stem cell transplantation (HSCT) is currently the only curative treatment for BMF. In patients with FA who do not have a matched sibling donor, HSCT conditioning regimens typically use reduced doses of cyclophosphamide, fludarabine and anti-thymocyte globulin (ATG) with total body irradiation (TBI) or busulfan. However, treatment is complicated by conditioning related toxicities, graft vs host disease (GvHD) and increased predisposition to malignancies later in life. Prior attempts to remove TBI from FA conditioning regimens have been unsuccessful due to increased risk of graft rejection - and alternative approaches have replaced TBI with busulfan which is still genotoxic.
Objective: To reduce acute and long-term treatment-related toxicities, we have developed a first of its kind treatment intended to improve the safety of allo-HSCT through: 1) a TBI- and busulfan-free conditioning regimen consisting of briquilimab, rabbit ATG (rATG - Thymoglobulin), fludarabine, cyclophosphamide and rituximab - briquilimab (formerly called JSP191) is a monoclonal antibody (mAb) that targets human CD117 to deplete host HSCs enabling blood and immune reconstitution with minimal toxicity with the other agents being used for transient immune suppression to prevent immunologic rejection; 2) transplantation of TCRαβ + T-cell/CD19 + B-cell hematopoietic grafts - a stem cell therapy that enhances donor hematopoietic and immune reconstitution while decreasing GvHD; and 3) a pre- and post-transplant monitoring protocol to maximize engraftment. This is the first HSCT regimen to use anti-CD117 mAb-based conditioning in immunocompetent patients without concurrent use of irradiation.
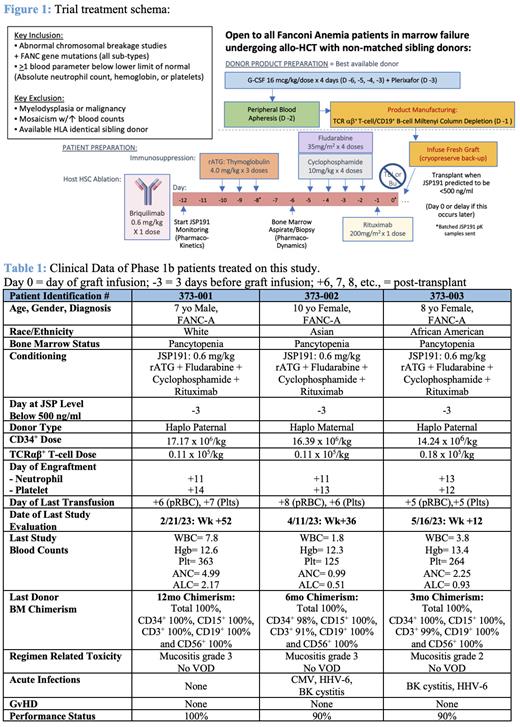
Methods: Patients with FA in BMF were eligible to be enrolled in this Phase 1b/2a trial to receive allo-HSCT with TCRαβ + T-cell/CD19 + B-cell depleted hematopoietic grafts from 10/10 unrelated, 9/10 unrelated or haploidentical family donors. Per the treatment schema, in this regimen a fixed dose of briquilimab was administered at 0.6 mg/kg in combination with standard FA dosing of rATG, fludarabine, cyclophosphamide and rituximab as lymphodepletion (Figure 1). Prospective pharmacokinetic measurements of briquilimab, rATG and fludarabine are included in the protocol.
Results: Treatment of all of the patients on the Phase 1b portion of the study has been completed with each of the three patients showing promising safety and efficacy results (Table 1). Importantly, briquilimab treatment was well tolerated without any complications with appropriate antibody clearance of < 500 ng/ml prior to HSCT in all three patients. Each of the patients achieved full blood count recovery by Day +14 post HSCT with haploidentical grafts that were within specifications of the protocol. Total, CD34 +, and CD15 + donor chimerism of > 98% in the bone marrow has been observed in all patients with > 91% chimerism in all immune subsets (CD3, CD19 and CD56) at the last follow-up with 1 year of follow-up post HSCT in the first patient. None of the patients have developed acute or chronic GvHD at the time of reporting.
Conclusions: All three patients in the Phase 1b portion of the study show early safety and efficacy of this approach. Briquilimab was well tolerated and all patients have shown prompt and durable donor engraftment. This data indicates that it might be possible to remove irradiation or alkylator chemotherapy from the conditioning regimen in patients with FA without matched sibling donors thus decreasing the chances for cancer predisposition in these patients that have inherited DNA repair defects.
Disclosures
Logan:Amgen, Autolus Therapeutics, Kadmon, Kite, Pharmacyclics, Talaris: Research Funding; AbbVie, Amgen, Actinium, BMS, Pfizer, Sanofi, Takeda: Consultancy. Boelens:Advanced Clinical: Honoraria; Immusoft: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Sobi: Consultancy, Honoraria; Omeros: Consultancy, Honoraria; Bluerock: Consultancy, Honoraria; Bluebird Bio: Honoraria; SmartImmune: Consultancy, Honoraria. Weissman:48 bio: Current equity holder in private company, Current holder of stock options in a privately-held company; Bitterroot bio: Current equity holder in private company, Current equity holder in publicly-traded company; Pheast bio: Current equity holder in private company, Current equity holder in publicly-traded company. Shizuru:Jasper Therapeutics: Consultancy, Current equity holder in publicly-traded company. Pang:Jasper Therapeutics: Current Employment, Current equity holder in publicly-traded company. Roncarolo:Graphite Biologics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; TR1X: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Atara: Membership on an entity's Board of Directors or advisory committees; Kamau Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Porteus:Graphite Biologics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Allogene Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Alaunos Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Kamau Therapeutics: Current equity holder in private company; CRISPR Tx: Current equity holder in publicly-traded company. Czechowicz:Rocket Pharma: Research Funding; Magenta Therapeutics: Current equity holder in publicly-traded company, Patents & Royalties; Jasper Therapeutics: Patents & Royalties, Research Funding; Decibel Therapeutics: Current equity holder in publicly-traded company; Editas Medicines: Current equity holder in publicly-traded company; Beam Therapeutics: Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal